|
Digital News Report – The U.S. Food and Drug Administration (FDA) approved the drug Ampyra (dalfampridine) extended release tablets for patients suffering with multiple sclerosis (MS).
The agency cited studies where Ampyra increased the walking speed of those treated with the drug compared to the placebo. “This is the first drug approved for this use,” the FDA said in their statement.
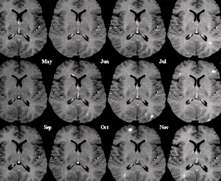
MS (aka disseminated sclerosis or encephalomyelitis disseminata) is a disease that eats away at the fatty myelin sheaths around the axons of the brain and spinal cord. More common in females, the disease is usually initially seen in young adults. It affects between 2 and 150 per 100,000.