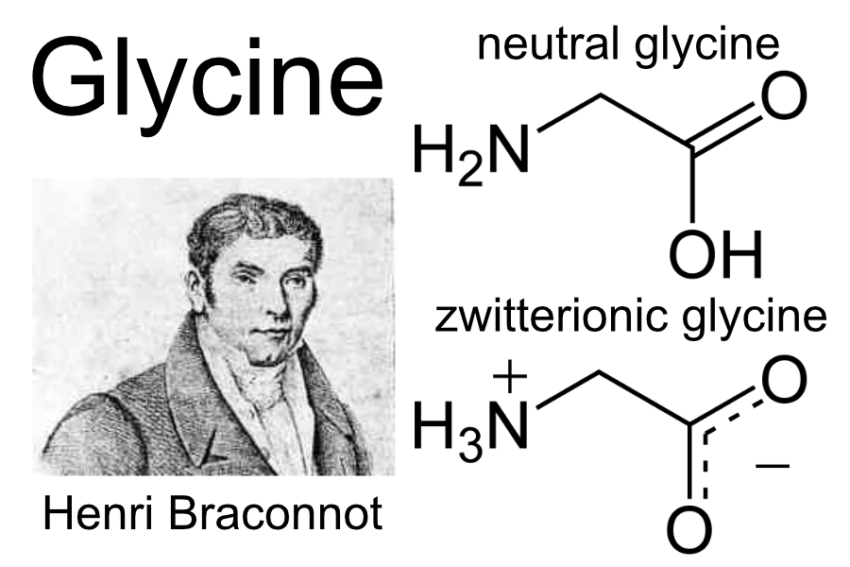
Glycine is an amino acid which are the building blocks of proteins. It is the simplest and smallest amino acid, with the chemical formula NH2-CH2-COOH. Glycine has a hydrogen atom as its side chain, making it non-polar and hydrophobic. It is also achiral, meaning it does not have a stereocenter and thus does not exhibit optical isomerism.
Glycine plays various roles in the body. It is involved in the synthesis of proteins, purines (building blocks of DNA and RNA), heme (a component of hemoglobin), and glutathione (an antioxidant). It also functions as an inhibitory neurotransmitter in the central nervous system, helping to regulate sleep, motor control, and other essential functions.
In addition to being synthesized by the body, glycine can be found in various food sources, including animal products such as meat, fish, and dairy, as well as plant-based sources like beans and nuts. It can also be taken as a dietary supplement.
Glycine has a sweet taste, which is why it is sometimes used as a flavor enhancer or sweetener in food products. The sweetness of glycine is less intense than that of common table sugar (sucrose), but it can still contribute a pleasant sweetness when used in appropriate quantities. In addition to its taste, glycine is highly soluble in water, which makes it easy to incorporate into various recipes and food products.
Etymology and History
The word “glycine” is derived from the Greek word “glykys” (γλυκύς), which means “sweet.”1 The amino acid was first isolated in 1820 by French chemist Henri Braconnot, who extracted it from gelatin by boiling it with sulfuric acid.2 Glycine was later synthesized for the first time by German chemist Adolph Strecker in 1858.3 Since its discovery, glycine has been the subject of extensive research, and its various roles and functions in biological systems have been elucidated over the years.
Physiological Functions
As a precursor to proteins, glycine is essential for the proper structure and function of many biological molecules. Its small size and non-polar nature allow it to fit into tight spaces within proteins, providing flexibility and stability to protein structures.
In collagen, the most abundant protein in the human body, glycine plays a crucial role in forming its characteristic triple helix structure.4 Collagen contains a high percentage of glycine, which appears in a repeating pattern along with other amino acids like proline and hydroxyproline. This specific sequence allows the formation of the stable triple helix structure that provides strength and resilience to connective tissues such as skin, tendons, and ligaments.
Glycine is encoded by all codons starting with GG: GGU, GGC, GGA, and GGG.5 This redundancy in the genetic code, also known as degeneracy, helps reduce the impact of mutations and ensures the accurate translation of genetic information into functional proteins.
Biosynthetic Intermediate
A biosynthetic intermediate is a molecule that participates in a biosynthetic pathway, which is a series of chemical reactions in living organisms that produce complex molecules from simpler precursors. Biosynthetic intermediates are formed during the process of biosynthesis, acting as intermediate compounds that are eventually converted into the final product or a subsequent intermediate in the pathway. These intermediates play a crucial role in the synthesis of various biomolecules such as amino acids, nucleotides, lipids, and carbohydrates.
Biosynthetic pathways are regulated by various mechanisms, including feedback inhibition, where the end product of a pathway inhibits an enzyme in an earlier step, thus controlling the overall production and maintaining the required balance of the biomolecules within the cell.6 Understanding these pathways and their intermediates can provide valuable insights into the functioning of biological systems and contribute to the development of therapeutic approaches for various diseases.
Glycine acts as a biosynthetic intermediate in several metabolic pathways, participating in the production of various biomolecules in the body. Some of the key roles of glycine as a biosynthetic intermediate include:
- Purine synthesis: Glycine is involved in the de novo synthesis of purines, which are essential building blocks of nucleic acids, such as DNA and RNA. In this process, glycine contributes to the formation of the purine ring structure found in nucleotide bases adenine and guanine.7
- Heme synthesis: Glycine takes part in the synthesis of heme, a critical component of hemoglobin, which is responsible for oxygen transport in red blood cells. In the first step of heme biosynthesis, glycine and succinyl-CoA condense to form δ-aminolevulinic acid (ALA), catalyzed by the enzyme ALA synthase.8
- Glutathione synthesis: Glycine is one of the three amino acids (along with cysteine and glutamate) that make up glutathione, a tripeptide with important antioxidant properties. Glutathione helps protect cells from damage caused by reactive oxygen species and plays a role in the detoxification of xenobiotics.9
- Creatine synthesis: Glycine participates in the synthesis of creatine, a molecule involved in energy metabolism in muscles and other tissues. The synthesis of creatine starts with the transfer of a guanidino group from arginine to glycine, forming guanidinoacetate, which is then methylated to produce creatine.10
Neurotransmitter
A neurotransmitter is a chemical substance that is released by neurons to transmit signals to other neurons, muscle cells, or gland cells, thereby facilitating communication within the nervous system. Neurotransmitters play a crucial role in the regulation of various physiological processes, including mood, cognition, sleep, appetite, and pain sensation, among others.
Neurotransmitters can be classified into two main categories based on their action:
- Excitatory neurotransmitters: These neurotransmitters increase the likelihood of generating an action potential in the receiving neuron, promoting the transmission of the nerve signal. Examples include glutamate, which is the primary excitatory neurotransmitter in the central nervous system, and acetylcholine, which is involved in muscle contraction and various cognitive functions.
- Inhibitory neurotransmitters: These neurotransmitters decrease the likelihood of generating an action potential in the receiving neuron, inhibiting the transmission of the nerve signal. Examples include gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the central nervous system, and glycine, which also acts as an inhibitory neurotransmitter, particularly in the spinal cord and brainstem.11
The balance between excitatory and inhibitory neurotransmitters is essential for maintaining proper nervous system function. Imbalances in neurotransmitter levels or their action can contribute to various neurological and psychiatric disorders, such as depression, anxiety, schizophrenia, and epilepsy.
Glycine functions as an inhibitory neurotransmitter in the central nervous system (CNS), particularly in the spinal cord and brainstem. When released from a presynaptic neuron, glycine binds to specific glycine receptors on the postsynaptic neuron. These receptors are ligand-gated ion channels that, upon activation by glycine, allow the influx of negatively charged chloride ions into the cell. This influx results in a hyperpolarization of the postsynaptic membrane, making it less likely for the neuron to generate an action potential, and thus inhibiting the transmission of the nerve signal.12
In addition to its inhibitory role, glycine also serves as a co-agonist at N-methyl-D-aspartate (NMDA) receptors, which are a subtype of glutamate receptors. Glycine binding to the NMDA receptor is necessary for the receptor’s activation in response to glutamate, an excitatory neurotransmitter. This dual role of glycine helps modulate excitatory neurotransmission and contributes to various functions such as learning, memory, and synaptic plasticity.13
Dysfunction or altered levels of glycine neurotransmission have been implicated in several neurological and psychiatric conditions, including epilepsy, schizophrenia, and chronic pain. Research into the modulation of glycine neurotransmission has led to the development of therapeutic strategies targeting glycine receptors or transporters to treat these disorders.14
Uses
Glycine has various uses and functions in the human body, in food, and in industrial applications. Here are some of its key uses:
- Protein synthesis: As an amino acid, glycine is a building block for proteins and is crucial for the proper structure and function of numerous proteins in the body, including collagen, which provides strength and resilience to connective tissues like skin, tendons, and ligaments.
- Neurotransmission: Glycine acts as an inhibitory neurotransmitter in the central nervous system, particularly in the spinal cord and brainstem, and as a co-agonist at NMDA receptors, which are involved in learning, memory, and synaptic plasticity.
- Biosynthetic precursor: Glycine serves as a biosynthetic intermediate in the synthesis of various biomolecules, including purines (building blocks of nucleic acids), heme (a component of hemoglobin), glutathione (an antioxidant), and creatine (involved in energy metabolism in muscles and other tissues).
- Food industry: Due to its sweet taste, glycine is sometimes used as a flavor enhancer or sweetener in food products. Its high solubility in water makes it easy to incorporate into various recipes and formulations.
- Pharmaceutical and cosmetic applications: Glycine is used as an ingredient in some pharmaceutical formulations, such as intravenous solutions and antacids, to improve solubility, stability, or taste. In cosmetics, it is used as a buffering agent, skin conditioning agent, or humectant.
- Dietary supplements: Glycine can be found in dietary supplements intended to support joint health, sleep, mental health, and exercise performance, among other purposes. Although the body can synthesize glycine, supplementation may be beneficial in certain situations or for specific populations.
- Chemical industry: Glycine is used as a starting material in the production of various chemicals, such as herbicides and other agrochemicals, as well as in the synthesis of more complex organic compounds for a range of industrial applications.
Recent Research
Serine and glycine metabolism in cancer
The research titled: “The ins and outs of serine and glycine metabolism in cancer,” focuses on the role of serine and glycine metabolism in cancer cells. Cancer cells reprogram their metabolism to support rapid, uncontrolled growth and survival in nutrient-poor conditions. While non-cancerous cells often have lower demands for serine and glycine, certain cancer subtypes show increased intracellular serine and glycine synthesis and rely on de novo production (production from simpler molecules within the body).
The article discusses how genetic alterations and copy-number amplifications of serine- and glycine-synthesis genes in cancer cells lead to high production and secretion of these metabolites, which support cancer development. Serine and glycine synthesis in cancer cells contribute to several processes that promote cancer progression, including:
- Enhancing macromolecule synthesis (building larger molecules from smaller ones)
- Neutralizing high levels of oxidative stress (which can damage cells)
- Regulating methylation (a process affecting gene expression) and tRNA formylation (a process involved in protein synthesis)
The authors also discuss the immunosuppressive potential of serine and glycine, which can help cancer cells evade the immune system. Additionally, serine and glycine are essential for the survival of non-transformed neighboring cells.
The article highlights emerging data on the possible moonlighting functions of serine- and glycine-synthesis enzymes, meaning these enzymes may have additional, previously unrecognized roles. Finally, the authors examine promising small molecules that target serine and glycine synthesis, which may provide new therapeutic strategies for treating cancer.
Citation: Geeraerts, S. L., Heylen, E., De Keersmaecker, K., & Kampen, K. R. (2021). The ins and outs of serine and glycine metabolism in cancer. Nature Metabolism, 3(2), 131-141. https://doi.org/10.1038/s42255-020-00329-9
Glycine and metabolic syndrome components
The research titled: “Effects of glycine on metabolic syndrome components: a review,” examined the role of glycine, a simple and abundant amino acid, in the development and treatment of metabolic syndrome components. Metabolic syndrome is a group of risk factors that increase the likelihood of developing heart disease, diabetes, and stroke. These risk factors include obesity, high blood pressure, high blood sugar, and abnormal cholesterol levels.
The authors conducted a literature search using Scopus, PubMed, and EMBASE databases to gather information on the relationship between glycine and metabolic syndrome. They found that the amount of glycine synthesized in the body is insufficient to meet metabolic demands in individuals with metabolic syndrome. Plasma glycine levels were found to be lower in subjects with metabolic syndrome compared to healthy individuals.
Interventions such as lifestyle modification, exercise, weight loss, or medication that improve metabolic syndrome symptoms also notably increase circulating glycine concentrations. The authors conclude that glycine supplementation can improve various components of metabolic syndrome, including diabetes, obesity, hyperlipidemia (high blood fat levels), and hypertension (high blood pressure).
In the future, the use of glycine may have significant clinical implications for treating patients with metabolic syndrome.
Citation: Imenshahidi, M., & Hossenzadeh, H. (2022). Effects of glycine on metabolic syndrome components: a review. Journal of Endocrinological Investigation, 45(5), 927-939. https://doi.org/10.1007/s40618-021-01720-3
Glycine has shown promise in various research areas for potential therapeutic applications. One such area involves its role as an inhibitory neurotransmitter in the central nervous system. Studies have found that glycine can modulate N-methyl-D-aspartate (NMDA) receptor function, which is implicated in numerous neurological disorders, including Alzheimer’s disease, Parkinson’s disease, and schizophrenia15. As a result, glycine and its modulators are being investigated as potential therapeutic agents for these conditions16.
Another area of interest is the potential anti-inflammatory and cytoprotective effects of glycine. Research has demonstrated that glycine can reduce inflammation and tissue damage in various experimental models of injury and disease, such as ischemia-reperfusion injury and liver injury17. These findings suggest that glycine could be a potential therapy for conditions where inflammation and tissue damage are involved18.
In addition, glycine has shown potential benefits for sleep and mental health. Some studies have found that glycine supplementation can improve sleep quality, reduce daytime sleepiness, and enhance cognitive performance19. This has led to interest in glycine as a potential treatment for sleep disorders and conditions associated with cognitive dysfunction20.
In summary, ongoing research on glycine holds promise for the development of therapies and potential cures in various areas, including neurological disorders, inflammation-related conditions, and sleep and mental health issues.
Glossary of Terms
- Alzheimer’s disease: A progressive neurological disorder that causes memory loss, cognitive decline, and behavioral changes.
- Amino acid: Organic compounds that serve as building blocks for proteins.
- Anti-inflammatory: A substance that reduces inflammation in the body.
- Biosynthetic precursor: A molecule that is used as a starting material in the synthesis of more complex molecules.
- Central nervous system: The part of the nervous system that includes the brain and spinal cord.
- Collagen: A structural protein found in connective tissues, such as skin, tendons, and ligaments.
- Cognitive dysfunction: Impaired mental function, including memory, attention, or problem-solving.
- Cytoprotective: A substance that protects cells from harmful agents or conditions.
- Diabetes: A group of metabolic disorders characterized by high blood sugar levels over a prolonged period.
- Glycine: A non-essential amino acid that serves as a building block for proteins, neurotransmitter, and precursor for other molecules.
- Hyperlipidemia: A condition characterized by abnormally high levels of fats (lipids) in the blood.
- Hypertension: A medical condition characterized by high blood pressure.
- Inhibitory neurotransmitter: A chemical substance that reduces the activity of neurons in the nervous system.
- Macromolecule synthesis: The process of building larger molecules from smaller ones.
- Metabolic syndrome: A group of risk factors that increase the likelihood of developing heart disease, diabetes, and stroke.
- Methylation: A process that involves the transfer of a methyl group to a molecule, often affecting gene expression.
- NMDA receptor: A type of glutamate receptor involved in synaptic plasticity, learning, and memory.
- Neurological disorder: A disorder affecting the nervous system, which includes the brain, spinal cord, and peripheral nerves.
- Obesity: A medical condition characterized by excessive body fat that increases the risk of various health problems.
- Oxidative stress: An imbalance between free radicals and antioxidants in the body, which can lead to cellular damage.
- Parkinson’s disease: A progressive neurological disorder characterized by motor symptoms, such as tremors and stiffness, as well as non-motor symptoms.
- Protein synthesis: The process by which cells build proteins from amino acids.
- Schizophrenia: A chronic and severe mental disorder characterized by abnormal thoughts, perceptions, and behaviors.
- Sleep disorders: A group of conditions that affect the ability to sleep well on a regular basis.
- tRNA formylation: A process that modifies transfer RNA (tRNA) molecules, involved in protein synthesis.
Footnotes
^1^ Liddell, H.G. & Scott, R. (1940). A Greek-English Lexicon. revised and augmented throughout by Sir Henry Stuart Jones. with the assistance of. Roderick McKenzie. Oxford. Clarendon Press.
^2^ Braconnot, H. (1820). Sur la conversion des matières animales en nouvelles substances par le moyen de l’acide sulfurique. Annales de Chimie et de Physique, 14, 173-178.
^3^ Strecker, A. (1858). Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper. Justus Liebigs Annalen der Chemie, 107(2), 151-162.
^4^ Shoulders, M. D., & Raines, R. T. (2009). Collagen structure and stability. Annual Review of Biochemistry, 78, 929-958.
^5^ Crick, F. H. (1966). Codon–anticodon pairing: The wobble hypothesis. Journal of Molecular Biology, 19(2), 548-555.
^6^ Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2002). Molecular Biology of the Cell (4th ed.). Garland Science.
^7^ Berg, J. M., Tymoczko, J. L., & Stryer, L. (2002). Biochemistry (5th ed.). W. H. Freeman.
^8^ Ponka, P. (1999). Cellular and molecular mechanisms of heme synthesis. Biochemistry and Cell Biology, 77(4), 297-304.
^9^ Wu, G., Fang, Y. Z., Yang, S., Lupton, J. R., & Turner, N. D. (2004). Glutathione metabolism and its implications for health. The Journal of Nutrition, 134(3), 489-492.
^10^ Brosnan, J. T., & Brosnan, M. E. (2007). Creatine: Endogenous metabolite, dietary, and therapeutic supplement. Annual Review of Nutrition, 27, 241-261.
^11^ Kandel, E. R., Schwartz, J. H., Jessell, T. M., Siegelbaum, S. A., & Hudspeth, A. J. (2012). Principles of Neural Science (5th ed.). McGraw-Hill Medical.
^12^ Betz, H. (1991). Glycine receptors: Heterogeneous and widespread in the mammalian brain. Trends in Neurosciences, 14(11), 458-461.
^13^ Johnson, J. W., & Ascher, P. (1987). Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature, 325(6104), 529-531.
^14^ Harvey, R. J., Yee, B. K., & GlyRα3, A. S. (2009). Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence, and pain. Nature Reviews Drug Discovery, 8(11), 866-885.
^15^ Traynelis, S. F., Wollmuth, L. P., McBain, C. J., Menniti, F. S., Vance, K. M., Ogden, K. K., … & Dingledine, R. (2010). Glutamate receptor ion channels: structure, regulation, and function. Pharmacological Reviews, 62(3), 405-496.
^16^ Paoletti, P., Bellone, C., & Zhou, Q. (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature Reviews Neuroscience, 14(6), 383-400.
^17^ Wang, W., Wu, Z., Dai, Z., Yang, Y., Wang, J., & Wu, G. (2013). Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids, 45(3), 463-477.
^18^ Zhong, Z., Wheeler, M. D., Li, X., Froh, M., Schemmer, P., Yin, M., … & Lemasters, J. J. (2003). L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Current Opinion in Clinical Nutrition and Metabolic Care, 6(2), 229-240.
^19^ Yamadera, W., Inagawa, K., Chiba, S., Bannai, M., Takahashi, M., & Nakayama, K. (2007). Glycine ingestion improves subjective sleep quality in human volunteers, correlating with polysomnographic changes. Sleep and Biological Rhythms, 5(2), 126-131.
^20^ File, S. E., Fluck, E., & Fernandes, C. (1999). Beneficial effects of glycine (bioglycin) on memory and attention in young and middle-aged adults. Journal of Clinical Psychopharmacology, 19(6), 506-512.