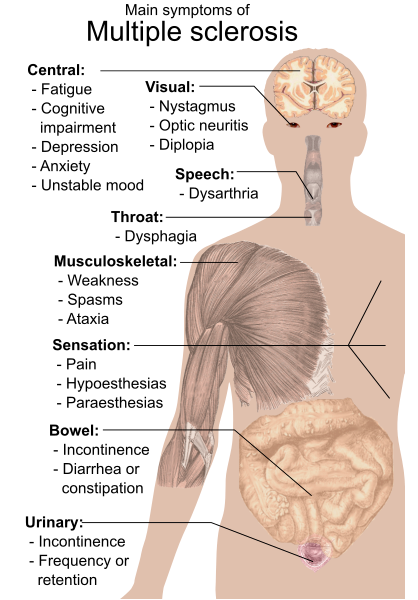
Multiple Sclerosis (MS)
Multiple Sclerosis (MS) is a complex and unpredictable neurological disorder that affects the central nervous system, disrupting the flow of information within the brain and between the brain and the rest of the body¹. MS is characterized by the immune system attacking the protective sheath (myelin) that surrounds nerve fibers, resulting in the formation of scar tissue, known as sclerosis². This process, known as demyelination, impairs the proper functioning of the nervous system, leading to a wide array of symptoms, including muscle weakness, fatigue, visual disturbances, and cognitive impairment³.
The precise cause of MS remains elusive, though researchers believe a combination of genetic and environmental factors contribute to its development⁴. The disease is typically diagnosed between the ages of 20 and 40, and affects more women than men⁵. While there is no definitive cure for MS, a range of disease-modifying therapies (DMTs) has emerged in recent years, offering hope for slowing disease progression and managing symptoms⁶. This article will delve into the current understanding of MS, explore potential risk factors, and discuss the latest advances in treatment and research.
Bruton Tyrosine Kinase Inhibitors (BTKIs)
Bruton Tyrosine Kinase Inhibitors (BTKIs) are a class of small-molecule drugs that selectively target the enzyme Bruton’s tyrosine kinase (BTK)⁷. BTK, a non-receptor tyrosine kinase, plays a critical role in B-cell receptor (BCR) signaling, which is essential for B-cell development, maturation, and activation⁸. Dysregulation of BCR signaling has been implicated in a variety of autoimmune disorders and B-cell malignancies, making BTK an attractive therapeutic target⁹.
BTKIs have demonstrated significant clinical efficacy in the treatment of B-cell malignancies, such as chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL)¹⁰. Ibrutinib, the first-in-class BTKI, was approved by the US Food and Drug Administration (FDA) in 2013 for the treatment of CLL and MCL¹¹. Since then, several other BTKIs, including acalabrutinib and zanubrutinib, have entered the market, expanding treatment options for patients¹².
In addition to their use in oncology, BTKIs are being investigated for their potential in the treatment of autoimmune diseases, such as rheumatoid arthritis and multiple sclerosis, where aberrant B-cell activity is implicated in disease pathogenesis¹³. This article will provide an overview of the mechanism of action of BTKIs, their current applications in the treatment of B-cell malignancies, and explore their potential in the management of autoimmune diseases.
B cells (B lymphocytes) and Microglia
B cells, also known as B lymphocytes, are a type of white blood cell that plays a crucial role in the immune system’s defense against pathogens¹⁴. They are responsible for producing antibodies, which are proteins that recognize and neutralize foreign substances such as bacteria, viruses, and toxins¹⁵. In addition to antibody production, B cells also function as antigen-presenting cells, which help initiate and regulate immune responses by presenting foreign antigens to other immune cells, like T cells¹⁶.
Microglia, on the other hand, are specialized immune cells found exclusively in the central nervous system (CNS)¹⁷. They act as the primary immune defense within the brain and spinal cord, protecting the CNS from infection and injury¹⁸. Microglia constantly survey their environment and respond to changes by removing damaged neurons, clearing cellular debris, and releasing inflammatory factors to initiate tissue repair¹⁹. However, when microglia become overactivated, they can contribute to the development of various neurological disorders, such as Alzheimer’s disease and multiple sclerosis²⁰.
In layman’s terms, B cells are immune cells that produce antibodies to help protect the body against foreign substances, while microglia are specialized immune cells in the brain and spinal cord that help maintain the health of the nervous system.
Targeting B Cells and Microglia: Unraveling the Promise of BTKIs for Multiple Sclerosis Management
The JAMA article discusses the potential of using Bruton Tyrosine Kinase Inhibitors (BTKIs) as a novel approach for treating Multiple Sclerosis (MS). Currently, disease-modifying therapies for MS target different aspects of the immune system, with a particular focus on T cells and B cells. The JAMA article ²¹emphasizes that while B cell depletion using CD20-targeting antibodies has shown success, long-term absence of B cells could pose safety risks, such as a reduction in the body’s ability to produce immunoglobulins (antibodies). As a result, researchers are exploring alternative B cell-directed therapies like BTKIs.
BTKIs target the Bruton Tyrosine Kinase enzyme, which is crucial for the activation of both B cells and myeloid cells like microglia. Different BTKIs vary in their binding mechanisms, selectivity, specificity, and ability to enter the central nervous system (CNS). Clinical trials using BTKIs in patients with relapsing-remitting MS and progressive MS have produced promising results in terms of efficacy and safety.
However, questions remain regarding the long-term effects of using BTKIs for treating inflammatory CNS diseases, changes in circulating antibody levels, and the potential of BTKIs to enter the CNS. Further research is needed to determine which BTKIs may be most suitable for different forms of MS and to clarify their role in the broader landscape of MS therapeutics²¹.
MS Therapies
There are several disease-modifying therapies (DMTs) currently approved for the treatment of multiple sclerosis (MS). These treatments can be categorized into injectable, oral, and infusion therapies. Some of the most commonly used DMTs include:
Injectable therapies:
- Interferon beta-1a (Avonex, Rebif)²²
- Interferon beta-1b (Betaseron, Extavia)²³
- Glatiramer acetate (Copaxone, Glatopa)²⁴
Oral therapies:
- Fingolimod (Gilenya)²⁵
- Dimethyl fumarate (Tecfidera)²⁶
- Teriflunomide (Aubagio)²⁷
- Siponimod (Mayzent)²⁸
- Cladribine (Mavenclad)²⁹
Infusion therapies:
- Natalizumab (Tysabri)³⁰
- Alemtuzumab (Lemtrada)³¹
- Ocrelizumab (Ocrevus)³²
- Rituximab (Rituxan)³³ – used off-label for MS
It is important to note that the choice of treatment depends on the specific type and severity of MS, as well as individual patient factors such as age, overall health, and personal preferences.
Glossary of Terms
- Alemtuzumab: A monoclonal antibody used as an infusion therapy for the treatment of MS, marketed under the brand name Lemtrada.
- Antibodies: Proteins produced by B cells that recognize and neutralize foreign substances such as bacteria, viruses, and toxins.
- Antigen-presenting cells: Immune cells that help initiate and regulate immune responses by presenting foreign antigens to other immune cells, like T cells.
- Avonex: A brand name for interferon beta-1a, an injectable therapy for the treatment of MS.
- Aubagio: A brand name for teriflunomide, an oral therapy for the treatment of MS.
- B cells: Also known as B lymphocytes, a type of white blood cell that plays a crucial role in the immune system’s defense against pathogens.
- Betaseron: A brand name for interferon beta-1b, an injectable therapy for the treatment of MS.
- Bruton Tyrosine Kinase (BTK): An enzyme centrally involved in the activation of B cells and myeloid cells like microglia.
- Bruton Tyrosine Kinase Inhibitors (BTKIs): A class of drugs under development that target the BTK enzyme, with potential applications in the treatment of MS.
- CD20: A surface molecule on B cells that is targeted by certain antibody-based therapies for MS.
- Central Nervous System (CNS): Comprised of the brain and spinal cord, the CNS is the primary site of inflammation and damage in MS.
- Cladribine: An oral therapy for the treatment of MS, marketed under the brand name Mavenclad.
- Copaxone: A brand name for glatiramer acetate, an injectable therapy for the treatment of MS.
- Dimethyl fumarate: An oral therapy for the treatment of MS, marketed under the brand name Tecfidera.
- Disease-Modifying Therapies (DMTs): Treatments that aim to modify the course of MS by reducing the frequency and severity of relapses and slowing down disease progression.
- Fingolimod: An oral therapy for the treatment of MS, marketed under the brand name Gilenya.
- Glatiramer acetate: An injectable therapy for the treatment of MS, also available under the brand name Glatopa.
- Gilenya: A brand name for fingolimod, an oral therapy for the treatment of MS.
- Immunoglobulins: Another term for antibodies, these proteins are produced by B cells and are essential for immune defense.
- Interferon beta-1a: An injectable therapy for the treatment of MS, also available under the brand name Rebif.
- Interferon beta-1b: An injectable therapy for the treatment of MS, also available under the brand name Extavia.
- Lemtrada: A brand name for alemtuzumab, an infusion therapy for the treatment of MS.
- Mavenclad: A brand name for cladribine, an oral therapy for the treatment of MS.
- Microglia: Specialized immune cells found exclusively in the central nervous system, responsible for protecting the brain and spinal cord from infection and injury.
- Multiple Sclerosis (MS): A chronic autoimmune disorder affecting the central nervous system, characterized by inflammation, demyelination, and axonal damage.
- Natalizumab: A monoclonal antibody used as an infusion therapy for the treatment of MS, marketed under the brand name Tysabri.
- Ocrelizumab: A monoc
- Ocrelizumab: A monoclonal antibody used as an infusion therapy for the treatment of MS, marketed under the brand name Ocrevus.
- Ocrevus: A brand name for ocrelizumab, an infusion therapy for the treatment of MS.
- Rebif: A brand name for interferon beta-1a, an injectable therapy for the treatment of MS.
- Relapsing-Remitting MS (RRMS): A type of MS characterized by episodes of worsening neurological function (relapses) followed by periods of partial or complete recovery (remissions).
- Rituxan: A brand name for rituximab, an off-label infusion therapy for the treatment of MS.
- Rituximab: A monoclonal antibody that targets CD20 on B cells, used off-label as an infusion therapy for the treatment of MS.
- Secondary Progressive MS (SPMS): A type of MS that initially follows a relapsing-remitting course but eventually transitions into a more steadily progressive phase, with or without occasional relapses.
- Siponimod: An oral therapy for the treatment of MS, marketed under the brand name Mayzent.
- Tecfidera: A brand name for dimethyl fumarate, an oral therapy for the treatment of MS.
- T cells: Also known as T lymphocytes, a type of white blood cell that plays a central role in cell-mediated immunity.
- Tysabri: A brand name for natalizumab, an infusion therapy for the treatment of MS.
- Teriflunomide: An oral therapy for the treatment of MS, also available under the brand name Aubagio.
- Primary Progressive MS (PPMS): A type of MS characterized by a gradual and continuous worsening of neurological function from the onset, without distinct relapses or remissions.
- Myelin: A fatty substance that insulates nerve fibers, allowing for efficient transmission of electrical impulses. Demyelination in MS disrupts nerve signal transmission and contributes to neurological symptoms.
Footnotes
1Compston, A., & Coles, A. (2008). Multiple sclerosis. Lancet, 372(9648), 1502-1517.
² Franklin, R. J., & Ffrench-Constant, C. (2008). Remyelination in the CNS: from biology to therapy. Nature Reviews Neuroscience, 9(11), 839-855.
³ Lublin, F. D., & Reingold, S. C. (1996). Defining the clinical course of multiple sclerosis: results of an international survey. Neurology, 46(4), 907-911.
⁴ Olsson, T., Barcellos, L. F., & Alfredsson, L. (2017). Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nature Reviews Neurology, 13(1), 25-36.
⁵ Tullman, M. J. (2013). Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. American Journal of Managed Care, 19(2 Suppl), S15-20.
⁶ Montalban, X., Gold, R., Thompson, A. J., et al. (2018). ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Multiple Sclerosis Journal, 24(2), 96-120.
⁷ Vetrie, D., Vorechovsky, I., Sideras, P., et al. (1993). The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature, 361(6409), 226-233.
⁸ Dal Porto, J. M., Gauld, S. B., Merrell, K. T., Mills, D., Pugh-Bernard, A. E., & Cambier, J. (2004). B cell antigen receptor signaling 101. Molecular Immunology, 41(6-7), 599-613.
⁹ Corneth, O. B., Verstappen, G. M., Paulissen, S. M., & de Bruijn, M. J. (2017). Enhanced Bruton’s tyrosine kinase activity in peripheral blood B lymphocytes from patients with autoimmune disease. Arthritis & Rheumatology, 69(6), 1313-1324.
¹⁰ Byrd, J. C., Furman, R. R., Coutre, S. E., et al. (2013). Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. New England Journal of Medicine, 369(1), 32-42.
¹¹ US Food and Drug Administration. (2013). FDA approves Imbruvica for rare blood cancer [Press release]. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-approves-imbruvica-rare-blood-cancer
¹² Woyach, J. A., Ruppert, A. S., Guinn, D., et al. (2018). BTK inhibition is effective in patients with CLL resistant to venetoclax. Blood, 132(Supplement 1), 3018-3018.
¹³ Di Paolo, J. A., Huang, T., Balazs, M., et al. (2011). Specific Btk inhibition suppresses B cell- and myeloid
¹⁴ Cooper, M. D., & Alder, M. N. (2006). The evolution of adaptive immune systems. Cell, 124(4), 815-822.
¹⁵ Janeway, C. A., Travers, P., Walport, M., & Shlomchik, M. J. (2001). Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science.
¹⁶ Batista, F. D., & Harwood, N. E. (2009). The who, how and where of antigen presentation to B cells. Nature Reviews Immunology, 9(1), 15-27.
¹⁷ Lawson, L. J., Perry, V. H., & Gordon, S. (1992). Turnover of resident microglia in the normal adult mouse brain. Neuroscience, 48(2), 405-415.
¹⁸ Hanisch, U. K., & Kettenmann, H. (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience, 10(11), 1387-1394.
¹⁹ Kettenmann, H., Hanisch, U. K., Noda, M., & Verkhratsky, A. (2011). Physiology of microglia. Physiological Reviews, 91(2), 461-553.
²⁰ Salter, M. W., & Stevens, B. (2017). Microglia emerge as central players in brain disease. Nature Medicine, 23(9), 1018-1027.
²¹ Dybowski, S., Torke, S., & Weber, M. S. (2023). Targeting B Cells and Microglia in Multiple Sclerosis With Bruton Tyrosine Kinase Inhibitors: A Review. JAMA Neurology, 80(4), 404-414.
²² PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. (1998). Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet, 352(9139), 1498-1504.
²³ Kappos, L., Polman, C. H., Freedman, M. S., et al. (2006). Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology, 67(7), 1242-1249.
²⁴ Comi, G., Filippi, M., Wolinsky, J. S., et al. (2001). European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis. Annals of Neurology, 49(3), 290-297.
²⁵ Kappos, L., Radue, E. W., O’Connor, P., et al. (2010). A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. New England Journal of Medicine, 362(5), 387-401.
²⁶ Gold, R., Kappos, L., Arnold, D. L., et al. (2012). Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. New England Journal of Medicine, 367(12), 1098-1107.
²⁷ O’Connor, P., Wolinsky, J. S., Confavreux, C., et al. (2011). Randomized trial of oral teriflunomide for relapsing multiple sclerosis. New England Journal of Medicine, 365(14), 1293-1303.
²⁸ Kappos, L., Bar-Or, A., Cree, B. A. C., et al. (2018). Siponimod