Multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and myasthenia gravis (MG) are three neurological disorders that affect different parts of the nervous system. MS is an autoimmune condition where the immune system attacks the myelin sheath surrounding nerve fibers, causing communication problems between the brain and body. ALS involves the progressive degeneration of motor neurons in the brain and spinal cord, leading to loss of voluntary muscle control. Myasthenia gravis is caused by faulty transmission of signals at the neuromuscular junction due to autoantibodies, resulting in muscle weakness and fatigue.
While these three diseases share some general symptoms like fatigue, vision problems, and mobility impairments, the underlying mechanisms and disease courses are distinct. MS follows a relapsing-remitting pattern for many patients, with flare-ups interspersed with recovery. ALS and myasthenia gravis typically follow a progressive decline. Diagnostic testing also differs, with MRI scans, lumbar punctures, and nerve conduction studies indicating MS, versus tests of motor neuron function for ALS and antibody blood tests for myasthenia gravis.
Treatments also vary, with MS managed through medications to reduce immune attacks and relapse severity. ALS has no cure and treatment focuses on minimizing symptoms. Myasthenia gravis can be controlled by medications that improve neuromuscular transmission. Understanding the differences between these three neurological conditions is important for early diagnosis and tailored treatment approaches for patients.
History:
Amyotrophic Lateral Sclerosis (ALS):
- First described in 1869 by French neurologist Jean-Martin Charcot, who conducted autopsies on patients and identified the degeneration of motor neurons.
- In the 1880s, it became known as Amyotrophic lateral sclerosis, referring to the combination of muscle atrophy and lateral sclerosis of the spinal cord.
- Research in the 1950s found that ALS runs in some families, suggesting a genetic component. The first gene identified was SOD1 in 1993.
Myasthenia Gravis:
- Reported by German physician Samuel Wilks in 1877, though earlier descriptions date back to the 1600s.
- In 1960s, researchers found autoantibodies interfering with acetylcholine receptors at neuromuscular junctions, defining the autoimmune basis.
- Treatments like cholinesterase inhibitors and immunosuppressants stemmed from this research.
Multiple Sclerosis:
- Early accounts of MS date back to the 14th century, but French neurologist Jean-Martin Charcot classified it as a distinct disease in 1868.
- In 1916, abnormalities in myelin were noted, implicating myelin damage in MS. MRI technology later confirmed lesions along myelinated fibers.
- Link to autoimmunity was established in 1960s, with autoantibodies and inflammation observed in nervous system tissue.
ALS
Amyotrophic Lateral Sclerosis (ALS) involves the progressive degeneration of motor neurons in the brain, brainstem and spinal cord. This leads to declining muscle control and eventual paralysis. Early symptoms often include muscle weakness or stiffness in the arms, legs, mouth or throat. More advanced stages cause difficulty speaking, swallowing and breathing. Diagnosis is made based on clinical evaluation, electromyography and nerve conduction studies.
Unfortunately there is no cure for ALS at this time. Riluzole is a medication that may modestly slow disease progression. Other treatments focus on minimizing symptoms and improving quality of life. This can include physical therapy to maintain mobility, occupational therapy to aid daily activities, speech therapy for communication, nutritional support, breathing assistance with a ventilator and medication to reduce pain, cramping and fatigue. Providing both physical aids and emotional support is crucial for ALS patients.
Some promising areas of research for ALS treatment include:
- Stem cell therapy – Introducing stem cells into the spinal cord to help replace lost motor neurons. Early clinical trials are underway.
- Gene therapy – Delivering healthy versions of defective genes, like SOD1, to target motor neurons. Shows potential in animal models.
- Antisense oligonucleotides – These synthetic strands can bind to RNA to lower production of mutant SOD1 protein. Being tested in human trials.
- Neurotrophic factors – Proteins that protect motor neurons. BDNF and VEGF show benefits in ALS models. Can be delivered directly to nervous system.
- Immunotherapy – Targeting autoimmune components may slow progression in some ALS patients. Plasma exchange and immunosuppressants are being studied.
- Combination therapies – Using multiple treatment strategies together may provide greater benefits than single drugs.
- Stem cell models – Deriving motor neurons from ALS patient stem cells helps screen promising drug candidates.
While there is no cure yet, intense research is underway to understand ALS and translate findings into clinical treatments. Bringing promising therapies through rigorous trials is crucial to establish safety and efficacy.
Myasthenia Gravis
Myasthenia gravis involves impaired communication between nerves and muscles, causing fluctuating and fatigable weakness. Symptoms most often affect the eyes, face, neck, and limbs. Eye-related issues like double vision and drooping eyelids are common. Muscle weakness increases with activity and improves with rest. Diagnosis relies on blood tests, nerve stimulation studies, and response to medication.
There is no cure, but several treatment options can help control symptoms. Cholinesterase inhibitors like pyridostigmine help improve neuromuscular transmission. Corticosteroids or immunosuppressants reduce autoimmune attacks. Plasmapheresis filters autoantibodies from the blood. Thymectomy surgery to remove the thymus gland may induce remission in some patients. Supportive care like physical therapy and assistive devices can also help preserve movement and independence. With treatment, most patients achieve good symptom control and improved quality of life.
Here are some promising areas of research for improving treatments for myasthenia gravis:
- Better autoantibody characterization – Understanding what initiates loss of self-tolerance and autoantibody development against acetylcholine receptors can guide targeted therapies.
- Novel immunosuppressants – Newer drugs like eculizumab can suppress immune attacks on neuromuscular junctions with potentially fewer side effects.
- Antisense oligonucleotides – These synthetic molecules can bind autoantibody RNA and reduce autoantibody production. Showing benefits in early studies.
- Stem cell transplants – Hematopoietic stem cells may help “reset” the immune system and induce long-term remission. Currently being tested.
- Gene therapy – Introducing normal copies of defective genes could correct issues with autoimmunity and thymus gland function.
- Removal of B-cells – Depleting B lymphocytes, which produce autoantibodies, may achieve remission in refractory cases.
- Enhanced diagnostics – New imaging and neuromuscular tests improve detection of impaired junctions to allow earlier treatment.
- Precision medicine – Matching therapies based on patient genetic profiles and disease subtypes could improve outcomes.
While current treatments can control myasthenia gravis, research advances may yield more targeted, curative options with fewer medication side effects.
Multiple Sclerosis
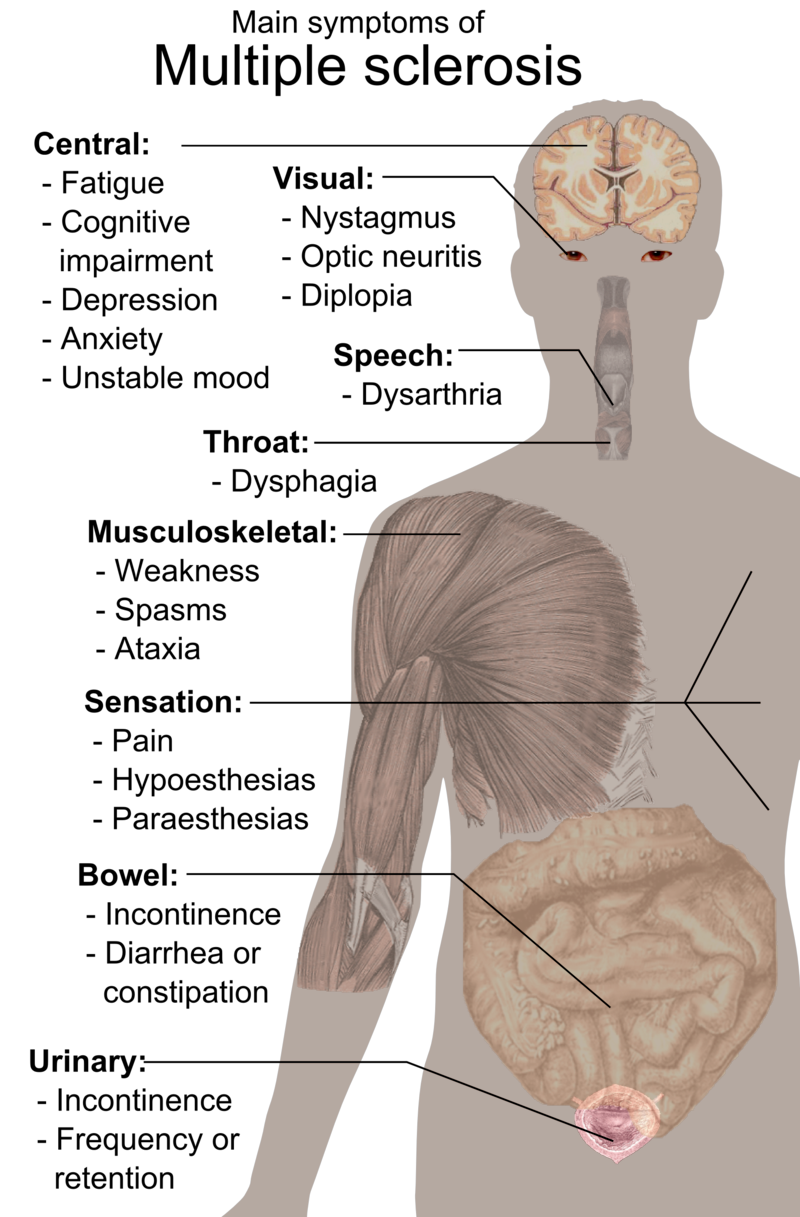
Multiple sclerosis involves gradual destruction of the myelin coating around nerve fibers in the central nervous system. This leads to disrupted neurological signaling, producing an array of symptoms. Early signs may include visual problems, numbness, tingling, and fatigue. Muscle weakness, mobility impairments, bladder issues, and cognitive changes often emerge over time. Symptoms vary by the location of myelin lesions and may come and go.
Diagnosis typically involves MRI, lumbar puncture, and ruling out other conditions. While there is no cure, medications and therapies aim to modify the disease course, speed recovery from attacks, and manage symptoms. Immunomodulating drugs like interferon beta and fingolimod suppress the immune system to reduce flare-ups and lesion formation.
Corticosteroids treat acute attacks. Physical, occupational and speech therapy preserve function. Some lifestyle measures like rest, exercise and stress reduction help as well. Recent stem cell and antibody treatments show promise in repairing myelin damage. With proper management, many patients with MS remain active and productive for decades after diagnosis.
Here are some promising areas of research for improving treatments and management of multiple sclerosis:
- Remyelinating therapies – Promoting repair of myelin sheaths around damaged nerves, such as through stem cell transplants or monoclonal antibodies.
- Neuroprotective approaches – Using drugs or molecules to protect nerves from inflammatory damage and slow disease progression.
- Biomarker development – Identifying biomarkers like proteins or mRNA that help predict treatment response and disease course.
- Progressive MS trials – Testing new drugs specifically for primary and secondary progressive MS, which have few treatment options.
- Gene studies – Identifying genes and risk factors that make people susceptible, to allow screening and personalized medicine.
- Virus link research – Investigating the potential roles of viruses like Epstein-Barr in triggering autoimmunity in MS.
- Lifestyle risk reduction – Optimizing vitamin D, obesity, smoking and other controllable factors to improve outcomes.
- Regenerative medicine – Using stem cell transplants, 3D bioprinting, and tissue engineering to heal damaged nervous system areas.
- Artificial intelligence – Applying AI and machine learning to analyze patient data and optimize therapy decisions.
While MS has no definitive cure yet, advanced research is building greater understanding of disease mechanisms and promising new treatment directions.
Image Credit: Public Domain. Symptoms of multiple sclerosis cs.svg wikimedia