Stroke – it’s a terrifying word and one that sends shivers down the spine of those who have experienced its devastating effects firsthand. In a blink of an eye, this deadly ailment can strike and disrupt the normal flow of blood to the brain, resulting in the death of brain cells. Time is of the essence when it comes to treatment, and doctors often turn to thrombolytic drugs, or “clot busters,” to dissolve the offending blood clot and restore blood flow.
In the world of thrombolytic agents, two names have emerged as front-runners: alteplase and tenecteplase. While both have been successful in their own right, questions still remain about which one is more effective and safer for patients suffering from a particular kind of stroke known as large vessel occlusion (LVO) stroke.
This article delves into the results of a clinical trial conducted across several Canadian stroke centers to compare the effectiveness and safety of these two drugs in treating LVO strokes. Researchers closely monitored patients treated with either drug and followed their recovery progress for 90 days to draw their conclusions. They were particularly interested in how many patients were able to regain their independence – a score known as the modified Rankin scale (mRS) score.
So, whether you’re a healthcare professional seeking to provide the best care for your patients, or someone who simply wants to understand the cutting edge of stroke treatment, join us as we explore the trial’s findings and what they could mean for future stroke care.
Understanding Stroke and Thrombolytic Therapy
To fully appreciate the implications of the study we’re about to explore, it’s important to understand the basics of stroke and the role of thrombolytic therapy in its treatment.
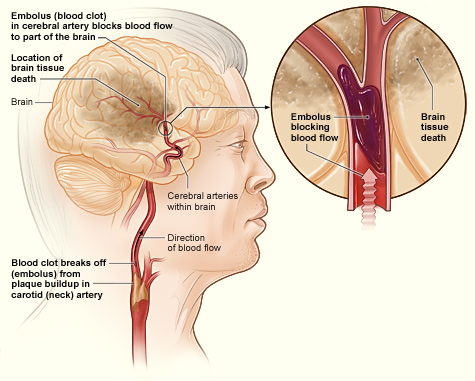
Stroke, one of the leading causes of death and disability worldwide, is a medical emergency that occurs when blood supply to a part of the brain is interrupted, often due to a blocked blood vessel (ischemic stroke) or a ruptured blood vessel (hemorrhagic stroke). This interruption in blood flow deprives the brain cells of oxygen and nutrients, causing them to die, which can result in lasting damage, disability, or even death.
One particular type of ischemic stroke we’re focusing on is large vessel occlusion (LVO) stroke. As the name suggests, LVO stroke involves blockage of one of the brain’s larger blood-supplying arteries. Due to the large area of brain tissue that can be affected, LVO strokes often result in severe symptoms and high rates of disability and death.
Enter thrombolytic therapy, often regarded as the frontline treatment for ischemic stroke. Thrombolytics, also known as “clot busters,” work by dissolving the blood clot that is blocking blood flow to the brain. The quicker a stroke patient receives thrombolytic treatment, the better the chances of reducing the severity of stroke-induced disability and improving recovery outcomes.
Two thrombolytic drugs that have gained significant attention in the management of ischemic strokes are alteplase and tenecteplase. Alteplase has long been the standard treatment option for acute ischemic stroke. However, tenecteplase, a newer drug, has sparked interest due to its ease of administration and potential advantages.
Despite this, until now, it has remained unclear which of these two drugs offers the greatest benefits in terms of safety and efficacy, particularly for LVO strokes. The study we’re discussing today aimed to shed light on this very topic.
Previous Investigations into Thrombolytic Therapy for Stroke
Before we delve into the current study, it’s important to contextualize it within the realm of previous research on thrombolytic therapy for stroke, particularly concerning alteplase and tenecteplase.
Alteplase has a long history as the standard thrombolytic treatment for acute ischemic stroke. The groundbreaking National Institute of Neurological Disorders and Stroke (NINDS) trial in 1995 provided strong evidence for its effectiveness. This trial found that patients who received alteplase within three hours of stroke onset were 30% more likely to have minimal or no disability three months later compared to patients who received a placebo.
However, alteplase isn’t without its limitations. It must be given as an infusion over an hour, which can delay treatment, and there are also concerns about its potential to cause bleeding in the brain.
Tenecteplase, a newer drug, has generated interest due to several potential advantages. For instance, it can be given as a single injection, potentially speeding up treatment. Furthermore, it has a higher affinity for fibrin, the protein that forms the structure of blood clots, which could potentially make it more effective at dissolving clots.
Some smaller studies and trials have provided promising evidence for tenecteplase. For example, a Norwegian trial published in 2012 found that tenecteplase was as safe as alteplase and suggested it might be more effective for treating ischemic stroke.
The 2018 EXTEND-IA TNK trial took it a step further, specifically investigating LVO strokes. This study found that patients treated with tenecteplase before undergoing mechanical thrombectomy (a procedure to physically remove the clot) had better reperfusion (restoration of blood flow) and functional outcomes than those treated with alteplase.
Despite these promising results, questions have remained. Larger-scale and more robust studies were needed to confirm these findings and further explore the relative safety and effectiveness of alteplase and tenecteplase, especially in patients with LVO strokes. This leads us to the current study in discussion – a prespecified secondary analysis of the ACT trial, one of the most comprehensive examinations of this subject to date.
The ACT Trial: Setting the Stage for a Showdown
The ACT (Alteplase Compared with Tenecteplase for Acute Ischaemic Stroke) trial, which this new study is based on, was initiated with the objective of advancing our understanding of thrombolytic therapy for stroke. Specifically, it aimed to address a pressing question in the medical community: Is tenecteplase as safe and effective as the tried-and-true alteplase for treating patients with acute large vessel occlusion (LVO) stroke?
Though earlier studies showed promising results for tenecteplase, they were often limited in size or scope. The ACT trial, however, enrolled a larger number of patients across multiple centers in Canada. It set out to provide a more comprehensive analysis, focused not only on the immediate impact of these drugs, but also the patient’s state of health 90 days after treatment. The hope was to ascertain whether one drug had an edge over the other in terms of safety and long-term recovery outcomes.
Key to this trial was the modified Rankin scale (mRS) score, a measure of disability often used in stroke research. A score of 0-1 signifies minimal to no disability, and a score of 0-2 denotes a level of disability that still allows the patient to be independent. By looking at these mRS scores at 90 days post-stroke, researchers hoped to gain insights into the true impact of tenecteplase and alteplase on patient recovery.
Thus, the ACT trial aimed to provide a more definitive answer to the tenecteplase versus alteplase question, in turn helping clinicians make more informed decisions when faced with an LVO stroke patient.
Design, Setting, and Participants of the ACT Trial
The ACT trial was meticulously designed to ensure robust, reliable results. Its foundation was a randomized clinical trial – a type of study considered the gold standard in determining the effectiveness of a treatment. This type of trial reduces bias and ensures that any differences observed are likely due to the treatment itself, rather than other variables.
The trial was conducted across 22 primary and comprehensive stroke centers in Canada. By spanning multiple centers, the study included a diverse range of patients and treatment settings, enhancing the generalizability of the results.
The trial enrolled patients who were 18 years and older and had experienced a disabling ischemic stroke within 4.5 hours of symptom onset – a time frame within which thrombolytic therapy is known to be most effective. For this analysis, the researchers focused on patients with LVO strokes, involving baseline occlusions in the intracranial internal carotid artery (ICA), M1-middle cerebral artery (MCA), M2-MCA, or basilar artery.
Patients were randomly assigned in a 1:1 ratio to receive either intravenous tenecteplase or alteplase. The tenecteplase group received a dose of 0.25 mg/kg, while the alteplase group received a standard dose of 0.9 mg/kg. This process of randomization further ensured that the comparison between the two drugs would be fair and unbiased.
In total, the trial enrolled 1,600 patients. Of these, 23 withdrew their consent, leaving a total of 1,577 patients. Among them, 520 (or 33.0%) had LVO strokes and were included in this prespecified secondary analysis.
In addition to monitoring the patients’ recovery over 90 days, the trial also measured the successful reperfusion (restoration of blood flow) after thrombectomy, a procedure for physically removing the clot, among patients who underwent the procedure.
With this design, setting, and participant pool, the ACT trial was well-positioned to shed light on the comparative safety and efficacy of tenecteplase and alteplase for LVO stroke treatment.
Exposure to Tenecteplase and Alteplase
In the context of clinical trials, ‘exposure’ refers to the treatment or intervention that participants receive. In the ACT trial, participants were exposed to either tenecteplase or alteplase, the two thrombolytic agents under comparison.
The patients in the tenecteplase group received a single intravenous dose of 0.25 mg/kg. This dosing regimen is notable because of its simplicity. Unlike alteplase, which requires an hour-long infusion, tenecteplase can be administered as a single injection. This advantage could potentially allow treatment to begin sooner, a critical factor given the time-sensitive nature of stroke treatment where every minute counts.
Considerations and Expectations
On the other hand, the patients in the alteplase group were given a dose of 0.9 mg/kg, which is the standard dosing regimen for this drug. Alteplase is typically given as an initial intravenous bolus (a quick, concentrated dose), followed by an infusion over the course of an hour.
The choice of using these particular dosages was based on prior research and established protocols. Both dosages have been proven safe and effective for their respective drugs. With this exposure, the study aimed to understand how these two different thrombolytic agents, administered in their standard manners, would compare in terms of safety and efficacy for treating acute LVO stroke.
Setting the Benchmarks: Main Outcomes and Measures
The ACT trial was designed to assess multiple outcomes, providing a comprehensive understanding of how tenecteplase and alteplase compare in their effectiveness and safety.
The primary outcome was the proportion of patients who had a score of 0-1 on the modified Rankin scale (mRS) at 90 days after stroke. The mRS is a measure of disability or dependence in daily activities, with a score of 0-1 indicating no significant disability. By focusing on this outcome, the trial aimed to understand which drug most effectively helped patients return to a normal life after a stroke.
Secondary outcomes of the trial included an mRS score of 0-2 at 90 days (indicating slight disability, but the patient is still able to look after their own affairs without assistance), mortality, and incidence of symptomatic intracerebral hemorrhage, a severe side effect that involves bleeding in the brain.
Additionally, the trial also measured angiographic outcomes in patients who underwent mechanical thrombectomy, a procedure to physically remove the blood clot causing the stroke. These outcomes included successful reperfusion (restoration of blood flow) on first and final angiographic acquisitions. This information helped the researchers understand whether either drug had an impact on the success of the clot removal procedure.
Multivariable analyses were also carried out, adjusting for factors such as age, sex, the severity of the stroke (as measured by the National Institute of Health Stroke Scale score), the time from stroke onset to needle (onset-to-needle time), and the location of the occlusion. These analyses ensured that the comparison between tenecteplase and alteplase took into account the potential influence of these other variables.
By evaluating these multiple outcomes and measures, the ACT trial aimed to deliver a detailed, nuanced view of the relative safety and effectiveness of tenecteplase and alteplase in the treatment of acute LVO strokes.
Breaking Down the Results: Tenecteplase vs. Alteplase
Out of the 1,577 patients involved in the ACT trial, 520 had a type of stroke known as a large vessel occlusion (LVO). This group was the primary focus of the study, and their results provide important insights into the effectiveness of tenecteplase and alteplase.
To simplify the results, let’s think of the “ideal outcome” after a stroke as being able to go back to daily life with little to no disability. In the trial, they measured this as having a score of 0-1 on a scale known as the modified Rankin scale (mRS). Three months after their strokes, around 33% of the patients in the tenecteplase group achieved this ideal outcome, compared to roughly 30% in the alteplase group. Although this might seem like a small difference, in the context of stroke treatment, even small improvements can be significant.
The study also looked at broader outcomes, where patients were still able to live independently, even if they had some disability (an mRS score of 0-2). Here, the results were very close, with about 49% in the tenecteplase group and 51% in the alteplase group achieving this outcome.
Safety is a critical consideration when assessing a stroke treatment, as the medication used to break down the blood clot can sometimes cause bleeding in the brain. In this trial, similar percentages of patients in both groups experienced this severe side effect. This suggests that tenecteplase is no more likely to cause bleeding than alteplase.
The trial also looked at death rates, and again, the results were quite similar between the two groups, showing no significant difference in mortality risk between tenecteplase and alteplase.
For patients who underwent a procedure to physically remove the blood clot, the trial checked whether blood flow was successfully restored. Here too, the results were similar between the tenecteplase and alteplase groups.
In essence, this study found that the two drugs performed quite similarly in terms of both effectiveness and safety. This suggests that tenecteplase, with its easier administration process, could be a viable alternative to alteplase in treating acute LVO strokes.
Conclusion and Relevance: A New Possible Pathway for Stroke Treatment
The ACT trial has shed light on an important question in stroke treatment. It examined whether tenecteplase, a drug that’s easier to administer, can be as safe and effective as the standard drug alteplase in treating patients with acute large vessel occlusion strokes.
The findings suggest that tenecteplase is indeed comparable to alteplase in terms of both safety and effectiveness. Patients treated with either drug showed similar levels of recovery, as measured by their ability to return to daily life with little to no disability. The rates of severe side effects, such as bleeding in the brain, were also similar between the two groups.
Importantly, these findings were consistent even when accounting for other factors that could influence outcomes, such as the patient’s age, the severity of the stroke, and the time from stroke onset to treatment.
What does this mean for patients and doctors? The results indicate that tenecteplase could be a viable alternative to alteplase for treating large vessel occlusion strokes. Given its simpler administration, tenecteplase might offer a more practical choice in certain settings or situations, potentially allowing treatment to begin sooner.
However, like all studies, the ACT trial has its limitations, and more research is always beneficial to confirm and expand upon these findings. Yet, this study takes a significant step forward in our understanding of stroke treatment, offering a new potential option that could improve the care and outcomes for stroke patients.
Citation:
Bala, F., Singh, N., Buck, B., Ademola, A., Coutts, S. B., Deschaintre, Y., … & Almekhlafi, M. (2023). Safety and Efficacy of Tenecteplase Compared With Alteplase in Patients With Large Vessel Occlusion Stroke: A Prespecified Secondary Analysis of the ACT Randomized Clinical Trial. JAMA Neurology.
Advance online publication. https://doi.org/10.1001/jamaneurol.2023.2094